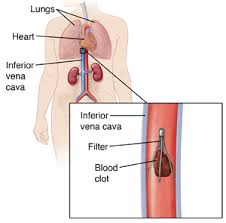
An inferior vena cava filter, or IVC
filter, is a tiny device implanted by doctors to “catch” migrating blood clots
that have broken away from an arterial wall. The device is often put in as a
prophylactic measure. In so doing, the IVC filter prevents a blood clot from
forming, preventing stroke and other life-threatening events.
The IVC filter is a wire device that is
often described as resembling a spider. Unfortunately, any one of the metal
extremities or shards on the device can break off and be carried away by the
blood stream, finding its way to the heart or lungs. The likelihood of
splintering increases the longer the device remains in the body. The devices
seem to work for their intended purpose, but should not be left in the body
permanently, and should be removed once the danger of a serious blood clot has
passed.
The main manufacturers named as
defendants in various lawsuits are C.R. Bard and Cook Group, Inc., or Cook
Medical.
It is alleged
that as early as 2003, Bard’s own research and studies showed that the IVC
filters posed an unreasonable danger to patients from splintering. The company did not tell the FDA
about their findings, or warn patients or doctors, and continued to sell and
market the Recovery IVC filter until it could create a suitable replacement.
Bard only removed the Recovery IVC from the market when it had a new filter,
the G2, in 2005. Since 2005, the FDA has received 921 device adverse event
reports involving IVC filters.
In May of last year, the FDA issued a
safety bulletin advocating that the ideal window for device retrieval is from
29 to 54 days after implantation. That said, the FDA was on record, as early as
2010, indicating that an IVC filter should be removed as soon as the danger of
a pulmonary embolism had passed.
In October 2014, the U.S. Judicial Panel
on Multidistrict Litigation consolidated 27 lawsuits against Cook Medical from
11 districts into Multidistrict Litigation No. 2570 in the Southern District of
Indiana before Judge Richard L. Young. This MDL is comprised mostly of lawsuits
over the Günther Tulip and Celect filters.
The first lawsuits filed against C.R. Bard were filed in California and Pennsylvania state courts in 2012. On August 18, 2015, the JPML centralized
22 lawsuits involving C.R. Bard IVC filters into Multi-District Litigation No.
2641 in Arizona under U.S. District Judge David G. Campbell. Those lawsuits
involve the Bard Recovery, G2, and G2
Express retrievable filters.
The use of an IVC filter may cause the
following problems:
IVC Filter migration
IVC Filter fracture
IVC Filter perforation
Tilting of the IVC Filter
The inability to retrieve the IVC Filter
Pulmonary embolism
Compromised respiration
Stroke
Death
Pittman, Dutton &
Hellums, P.C. is currently investigating IVC filter cases. If you or someone
you know received an IVC filter and has been injured, please contact Booth
Samuels at (866) 515-8880 or by email at booths@pittmandutton.com